Read this blog in:
Protein purification, type IV pili and integrative structural biology – an update from Paris
Time flies by very quickly in the world of science. You start your PhD and suddenly the first year have passed. Initially, when moving away from the safe surroundings of your homeland, the time is being spend trying to adapt to the new cultural impressions, building professional and social networks, and last but not least getting settled in a new lab with a new exciting project. After one year many of the challenges have been overcome and new insights have been gained both professionally and personally.
When I was going into science and biochemistry, I was often told that experiments will have a success rate of 10%. Therefore, you are looking for much more failure than success when conducting experiments. Maybe not complete failure, but your results turn out to be different than what you had hypothesised. In the initial part of a structural biology project, one need to produce and purify reasonable amount of protein, before further investigations can be conducted. If the obtention of the protein is causing troubles, the project seems to lag and the utilisation of more exciting and sophisticated experiments and analysis are far away in the future. This have been the case for my project. But the skies seem to have cleared from the sky and a sunbeam is shining brightly on my purification in the lab.
This means that the further investigations have moved a lot closer. My spirits have started to rise again as the main purpose of the project will come a bit closer. The title of my PhD project describes what is still to come: “An integrative strategy for structure determination of bacterial type IV pili”.
Type IV pili
The bacterial type IV pili is a proteinaceous polymers that are extended from the bacteria to perform a biological function. The biological function of type IV pili is very versatile and can range from facilitating twitching motility, DNA uptake and also promote infection of certain pathogen bacterial species. A very elaborate protein machinery is assembling the pili. This is located in both the inner and outer membrane of Gram-negative bacteria. The machinery can actively elongate and retract the pili.
The pili itself is composed of thousands of building block proteins, called pilins, and can be extended to reach a length of several µm. The pilins are divided into two groups, major and minor pilins. The major pilin is a single protein which have a much higher abundance compared to the minor pilins. The major pilin are forming the bulk of the pilus. The minor pilins are much lower abundant, but are very important for the biological function and even for the formation of the pili. My project is focusing on the determination of the structure of these minor pilins and their interaction. This work is an extension of the work previously performed in the lab where the structure of the pili composed of the major pilin was determined.
Integrative structural determination
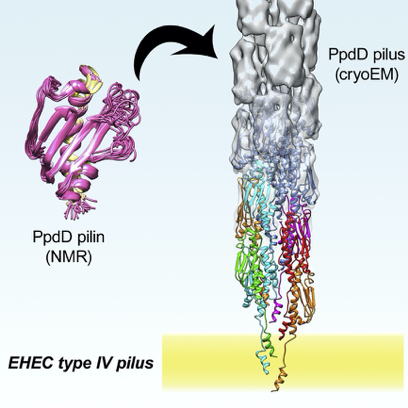
The NMR structure of PpdD is modelled into the density map of the cryo electron tomogram.
The ViBrANT PhD consortium had agreed on the 15 ESRs writing a mini-review for a special issue of Medical Microbiology and Immunology. This was a very nice opportunity to get into the literature and gain depth knowledge of the background for the project. From reading a lot of structural based articles it has been very clear that structural determination of pili requires a lot of different structural techniques. The structure of the individual pilins can be solved using NMR or X-ray crystallography. When utilising cryo-EM a low-resolution structures of the pili can be obtained. Furthermore, information about protein-protein interactions, stoichiometry, ligand binding etc. can be obtained with different techniques. The structure of the assembled pilus can then be modelled by combining these experimental data.
The working hypothesis
From homologous pili systems the minor pilins are thought to interact to form a stable complex which should initiate the assembly of the pilus. That was shown for other systems. During my PhD, I will investigate this hypothesis in our system in E. coli. I will map the interactions of the minor pilins and determine the structure of the minor pilins.
Secondment in Tübingen
As a part of ViBrANT we have the possibility to visit and work in other labs from the consortium. In this regard I spend two months in Tübingen in the lab of Thilo Stehle at the Interfakultäres Institut für Biochemie. Tübingen is a lovely little city where the nature is just outside the door and the city is full of students. When I arrive in Germany I was still struggling with the purification of the pilins. It was very useful to have new eyes on the project giving feedback and advice. Even though, the secondment did not result in major breakthrough regarding the research, it was a very nice experience to visit and work in another lab. The settings for my stay in Tübingen was also accompanied by Christmas lights and atmosphere which lifted the spirits in the cold and dark winter.

Beautiful settings by night in the city of Tübingen.
In the end of the secondment I went on a trip to the Swiss Light Source at the Paul Scherrer Institut in Switzerland. This was a great insight into the data collection of X-ray crystal structures.
Public outreach

Iris was visiting the lab, here we are waiting to evaluate the gel running in the background.
During the first week of February a high school student was visiting our lab in Paris. We showed her the basic principles of protein purification. Furthermore, we tried to engage her in all the exciting things you can do with modern science and technologies. Among others I showed her how to visualise proteins by SDS-PAGE and the basic principles of affinity chromatography and size exclusion chromatography.



